In This Section
- Home
- Semester and Timetable Information
- Study Physics
- Our Research
- Our People
- Careers and Alumni
- Seminars, News and Events
- Outreach and School Resources
- About the School
- What is Physics
- The Crawford Observatory
- Frequently Asked Questions
- UCC Futures Quantum & Photonics
- Supports/EDI
Boyle’s law states that for a fixed mass of an ideal gas at constant temperature, its pressure (P) and volume (V) obey the relationship
PV = constant
In other words, if an ideal gas is confined in a container whose volume can be changed by changing the pressure, then the pressure should be inversely proportional to the volume, that is
P ∝ 1/V
Thus, if a particular gas obeys Boyle’s law, a graph of pressure against the reciprocal of the volume should be a straight line passing through the origin.
 |
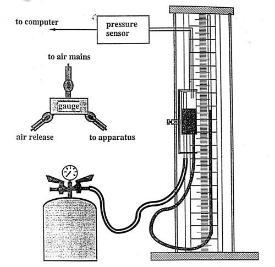 |
The apparatus used in this experiment is shown above. The sample of gas under investigation is the mass of air trapped in the closed glass tube in the right hand arm. The glass tube has uniform cross-sectional area and thus the volume of the trapped air is proportional to the length of air in the tube. This can be measured using the scale fixed to the apparatus. The pressure of the trapped air can be varied by altering the air pressure above the water level in the left-hand arm, which can be moved up and down to equalise the water levels in the two arms of the apparatus. When the water surfaces are in the same horizontal plane the pressure of the trapped gas is the same as the air pressure in the reservoir. This, in turn, can be measured on the attached computerised pressure gauge.
The pressure can be changed by increasing or decreasing the pressure of the air in the attached reservoir which can be connected to the compressed air supply in the laboratory.
If you want to explore the concepts of pressure and volume try you hand with these online labs / simulations:
School of Physics
Scoil na Fisice
Contact us
Room 213 (Physics Office), 2nd floor, Kane Science Building, University College Cork, Ireland.,
