Fingolimod’s double-edged sword effects on T cell regulation in brain ischaemia may explain inconsistent improvement in experimental stroke studies.
Fingolimod (FTY720, Gilenya) is an FDA- and EMA-approved drug for the treatment of multiple sclerosis (MS). Due to commonalities between MS and stroke pathophysiology, this drug has been trialled in ischaemic stroke. Fingolimod has generally, but not always, shown neuroprotective effects in stroke models. Effects unrelated to fingolimod’s primary mechanism of inhibiting lymphocyte trafficking are also likely to be involved in its mechanism of action
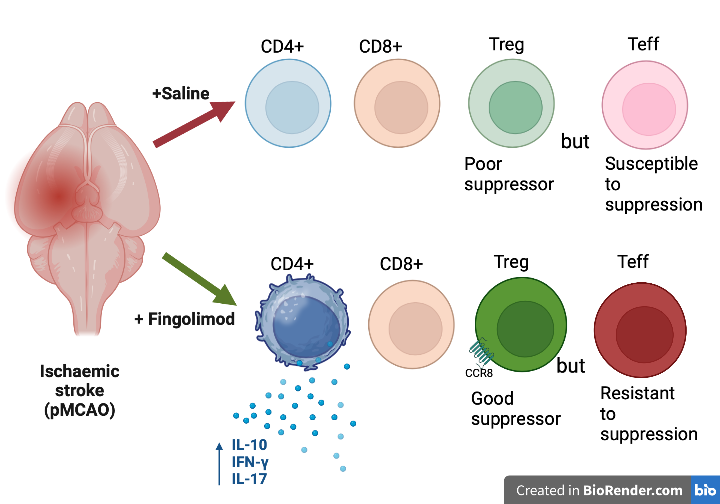
In a recent collaborative study published in the European Journal of Immunology, Drs Kyle Malone and Christian Waeber (Department of Pharmacology and Therapeutics), Jennifer Shearer (School of Pharmacy) and Anne Moore (School of Biochemistry and Cell Biology) examined the effects of fingolimod on regulatory T cell mechanisms in a murine stroke model. Fingolimod treatment increased CD4+ T cell cytokine production and CCR8 receptor expression on Treg. Notably, fingolimod improved the suppressive function of Treg post-stroke while also increasing the resistance of CD4+ effector cells to this suppression. Fingolimod’s capacity to increase both effector and regulatory functions may explain to the lack of consistent improvement in functional recovery in experimental brain ischaemia.
