by Bianca Govi, School of BEES, University College Cork
Sunlight is vital for life on Earth, as -through photosynthesis- it provides the energy all food chains ultimately rely on. Light also gives surface dwelling organisms the ability to sense the environment around them, but not all perceive light in the same way. Some, like humans, have complex eyes, but even plants use light as information.
What actually is light?
Light, a manifestation of the electromagnetic force – one of the fundamental forces of the universe, is energy propagating through space as ‘waves’. The electromagnetic spectrum is composed of waves of different (wave)lengths, oscillating at different frequencies, forming a continuum that contains the ‘visible light’ that we can see, and extends on the one side into infrared (heat) and radio waves, and on the other into ultraviolet and ionizing radiation. Colour, wavelength and energy (usually measured in electronvolts [eV]), are intrinsic qualities of waves oscillating at a given frequency (Fig. 1).
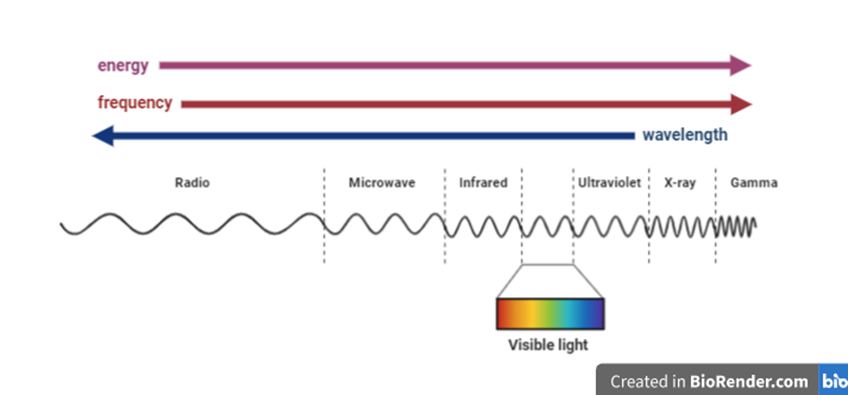
All waves travel at the same maximal speed (known as the ‘speed of light’ or c)1, but different wavelengths behave differently when encountering a medium boundary (such as a glass surface) at an angle. This ‘dispersion’ enables the splitting of the beam by wavelength, a process first scientifically investigated by Isaac Newton in the 1660s. Using a prism, Newton separated “white” light into its components, revealing what he identified as the seven visible colours: red, orange, yellow, green, blue, indigo, and violet (Fig. 2).
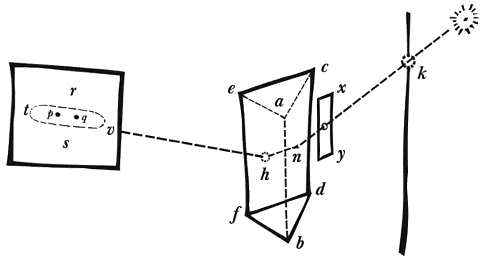
The study of “invisible light” began in 1800, when German-British astronomer Frederick William Herschel discovered infrared radiation by measuring the temperature in the space just beyond the red dispersion beam of a prism. The following year, German chemist Johann Wilhelm Ritter described “chemical rays”, now known as ultraviolet, at the opposite edge of the visible spectrum – just beyond the visible violet strip.
How do we measure light intensity?
The quality of light is defined by its wavelength colour/frequency/energy. But what about quantity? There are several ways of measuring and expressing the amount of light received by a surface. An important first distinction is between photometry (perceived brightness) and radiometry (energy transferred). Photometry deals with the collection of measurements that describe brightness as experienced by the human eye, which is not equally sensitive to different wavelengths. Quantities like luminous flux (Lumen) and luminous intensity (candela) that are used to describe indoor lighting layouts belong to this system2. Radiometry, in contrast, is concerned with absolute radiation energy, irrespective of our ability or inability to directly perceive it (Fig. 3).
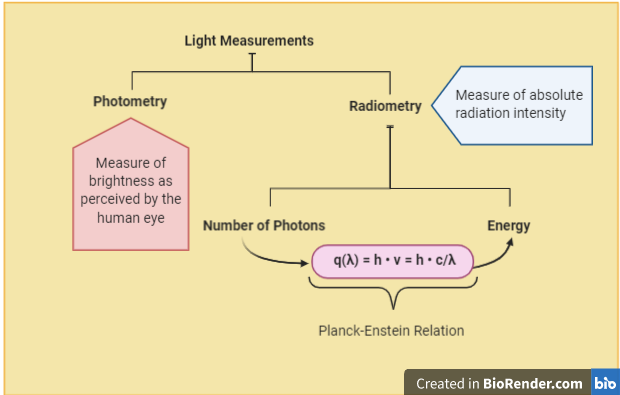
Instruments used to measure light energy intensity fall into three main types: radiometers, spectrometers, and spectroradiometer. Radiometers commonly employ photodiodes to measure total incoming irradiance across broad wavelength ranges3. Spectrometers diffract the incoming beam into its constituent wavelengths (by way of a prism or diffraction grating surface) and produce measures of relative intensity as a function of wavelength. Spectroradiometers are calibrated spectrometers that associate an absolute intensity measurement to each wavelength. The units of this measurement can either relate to quantum flux density (more about this later) or to power per unit area [e.g. Watts per square metre].
Given the dual wave/particle nature of electromagnetic radiation, the energy transferred from a radiating source to a receiving surface is not a continuum but is delivered in quanta of energy called photons or light particles. Short wavelength photons carry more energy than longwave ones: for example, 280 nm photons (shortwave ultraviolet-B) carry about twice the energy (Wm‑2) of the same number of visible green photons (~ 560 nm). The spectrum segment between 400 nm (blue) and 700 nm (red) is referred to by plant scientists as ‘photosynthetically active radiation’ (PAR), as photosynthetic reactions are driven by quantum flux of photons within this range4. As it takes a certain number of photons to drive the multiple steps in the photosynthetic fixation of one CO2 molecule, the number of incident photons per area per unit time (the ‘photon flux density’) is how light intensity is commonly expressed in plant biology.
Light quality and quantity from a plant’s perspective
PAR as a homogeneous block is, however, a simplification. Not all PAR wavelengths contribute equally to photosynthesis, while wavelengths beyond the visible also impact on plant photosynthetic efficiency as well as adaptive responses. The effect of light on biological systems depends on both its quality and quantity, so the intensity distribution over the entire relevant spectrum (‘spectral irradiance’) is the best way of describing light environments. However, full spectral curves are usually impractical to handle in data analysis, so simplifications (integrations and ratios) are commonly used in photobiology.
Variations in the spectrum can have surprisingly complex effects on plant biology. For instance, in addition to the varying efficiency by which different wavelengths within PAR drive photosynthesis, how energy is distributed over the spectrum has a conspicuous role in plant development and acclimation. Apart from the photosynthetic machinery, plants possess sensing mechanisms (photoreceptors) that enable them to use light quality as cues about their environment. For instance, cryptochrome (blue-sensitive photoreceptors) drive phototropism (i.e. growth towards light source), while phytochromes (red:far-red) regulate germination and elongation. Ultraviolet radiation, besides having the potential to create DNA damage, is also sensed by a photoreceptor named UVR8, and can alter plant morphology and metabolic profile. This can be thought of as plants “seeing” ultraviolet and reacting to the information it provides. Plant ultraviolet research opens up new questions and possibilities about what the most important aspects of light environment are from a plant perspective.
Awareness of how light regimes affect plant development and performance has gained new practical importance for the optimization of sheltered agricultural systems: greenhouses and more recently vertical farming. This is where UV-SINTEC comes in: in our project, we aim to contribute to the understanding of how the light environment, in its quantity and quality, affects plant growth and development. We will use this knowledge to optimize indoor horticulture and improve food nutritional properties. Which brings us back to our original question: How many plant biologists does it take to change a light bulb? Only one, but many more to discuss which bulb to get.
Footnotes:
1. The first to provide proof that light is not instantaneous was astronomer Ole Christensen Rømer, by observation of inaccuracies in tables predicting eclipses of the moons of Jupiter (tables that had been proposed by Galileo Galilei as a method for calculating longitude).
2. The sensitivity of the human eye is, however, not typical of the visual ranges across the animal kingdom: insects, as well as some birds and fishes, have eyes that can perceive ultraviolet radiation. As a result, the patterns on flowers are often more striking in the ultraviolet, as they evolved to be easily located by pollinators. The image below shows, from left to right, a ranunculus flower as seen through visible light, ultraviolet, and infrared.
_Vis_UV_IR_comparison-700x350.jpg)
3. An early radiometer, or “light mill”, was developed in 1873. The wheel spins thanks to the heating of air close to the exposed vane plates’ surface and its subsequent flow towards the cooler side. The movement was initially incorrectly believed to be a direct proof of radiation pressure, but the vanes will not spin in a complete vacuum.

4. PAR is not a uniform block in terms of photosynthetic efficiency. In 1971, physicist Keith McCree measured photosynthetic activity against irradiance at different PAR wavelengths. His experiment showed that, per absorbed irradiance, different PAR wavelengths displayed different efficiencies in driving CO2 assimilation.
Bibliography
- The Newton Project, MS Add. 3975, pp. 1-22, Cambridge University Library, Cambridge, UK
- Aphalo, P. J., Albert, A., Björn, L. O., McLeod, A., Robson, T. M. and Rosenqvist, E. (eds.) 2012. Beyond the visible: A handbook of best practice in plant UV photobiology. COST Action FA0906 UV4growth, 176 pp. Open access at the repository of the University of Helsinki: http://hdl.handle.net/10138/37558
- Wikipedia “Electromagnetic spectrum” page: https://en.wikipedia.org/wiki/Electromagnetic_spectrum
- Spectrometer, spectro-radiometer, spectro-photometer, photometer, radiometer. What is the difference? https://alliedscientificpro.com/blog/welcome-to-our-blog-1/post/spectrometer-spectro-radiometer-spectro-photometer-photometer-radiometer-what-is-the-difference-68
- Lind O, Mitkus M, Olsson P, Kelber A. Ultraviolet vision in birds: the importance of transparent eye media. Proc Biol Sci. 2014; 281(1774): 20132209. https://doi.org/10.1098/rspb.2013.2209
- Hoover, William H., 1937. “The dependence of carbon dioxide assimilation in a higher plant on wave length of radiation.” Smithsonian Miscellaneous Collections. 95 (21):1–13. https://repository.si.edu/handle/10088/23987
- Pattison, P.M., Tsao, J.Y., Brainard, G.C., Bugbee, B., 2018. LEDs for photons, physiology and food. Nature 563, 493–500. https://doi.org/10.1038/s41586-018-0706-x
- How many photons does it take to make a cyanobacterium on Cell Biology by the Numbers: http://book.bionumbers.org/how-many-photons-does-it-take-to-make-a-cyanobacterium/
