Research ethics approval is required for all research involving direct and indirect interaction with human participants (via computer / internet, in clinical or other settings) and/or which uses personal data of identifiable individuals. It is also required for any research that involves the use of animals.
It is the responsibility of all researchers (regardless of the nature of their research) and all supervisors of student research (undergraduate and postgraduate) to ensure that research is carried out in an ethical manner and to adhere to all associated legal requirements.It is the responsibility of all researchers and research supervisors in UCC to ensure adherence to the UCC Code of Research Conduct. Formal prior ethics approval by a recognised UCC Research Ethics Committee (REC) is required.
Explore This Page
When do I need to obtain research approval?
Research ethics approval must always be obtained in advance of carrying out the research and ethical approval will not be granted retrospectively. Only when you obtain approval for your research, can you start that part of the research, which directly/indirectly involves people or their data.
Please make sure that you give yourself a good lead-time in applying for ethics approval.
University Ethics Committees
There are three sub-committees of the University Ethics Committee (UEC); Clinical, Animal, and Social.
What research ethics committee should you apply to?
Clinical Research | Animal Experimentation | Social Research
Clinical Research
If the research project is clinical in nature, then it must be referred to the Research Ethics Committee of the Cork Teaching Hospitals (CREC). The requirements of CREC are set out in the CREC manual which is available from its secretariat. In broad terms, prior approval is necessary where the research methodology involves:
- Therapeutic interaction with human participant(s).
- A clinical trial of, inter alia, a medical device, medicinal product, or clinical technique.
- Development of diagnostic techniques using human participants.
- Access to, or utilisation of, human tissue and body fluids.
- Access to, or utilisation of, identifiable medical data concerning individuals (such as clinical records) by parties not directly concerned in the provision of care to these individuals.
- Interaction with / observation of individuals in a healthcare context or setting.
Email contact: crec@ucc.ie
Animal Experimentation
Research which involves experimentation on animal subjects must be approved by the Animal Experimentation Ethics Committee (AEEC) with input from the Animal Welfare Body. This is a prerequisite to obtaining the necessary authorisations for animal experimentation as prescribed by law. If seeking to apply for or renew an existing animal experimentation authorisation, the researcher must first refer to the Animal Welfare Body and Director of the Biological Services Unit.
Email contact: awb@ucc.ie
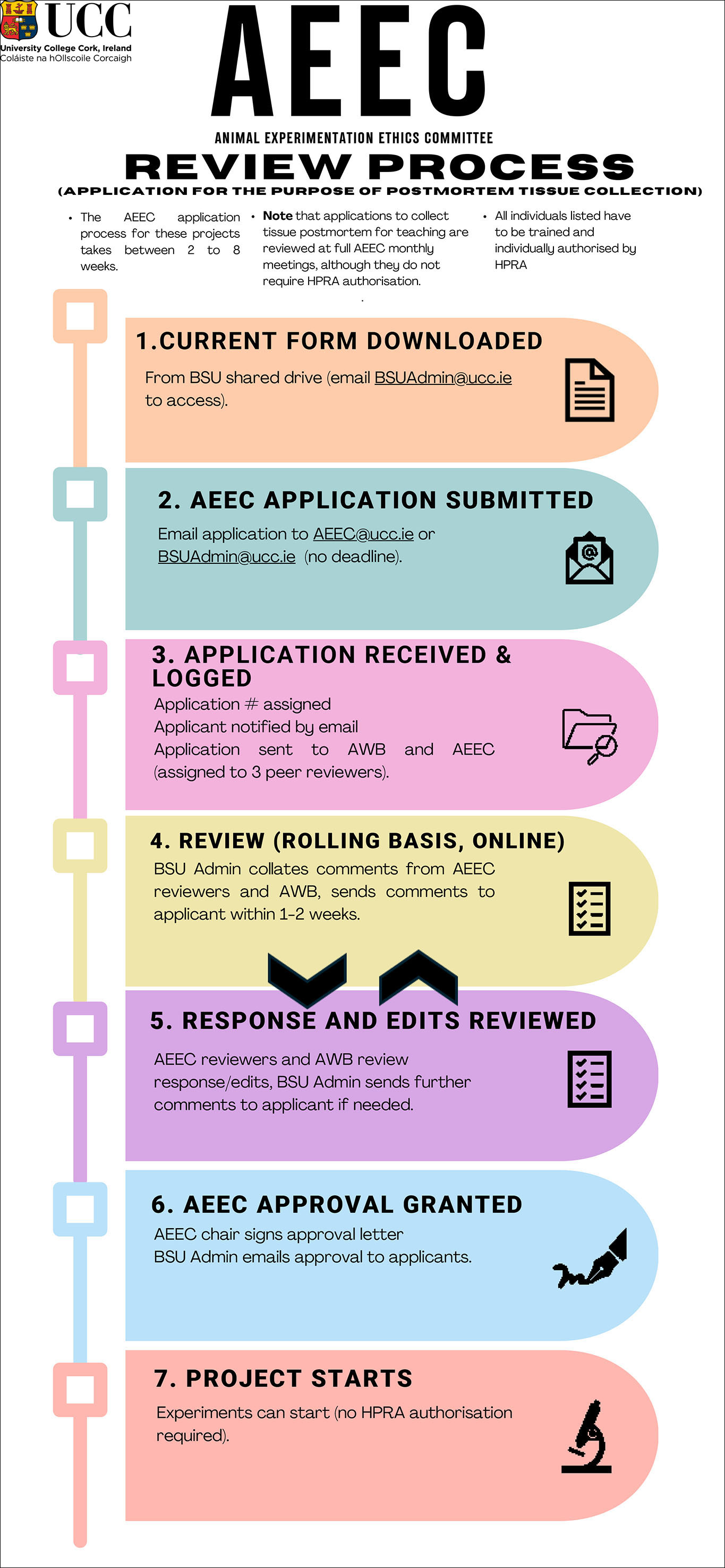
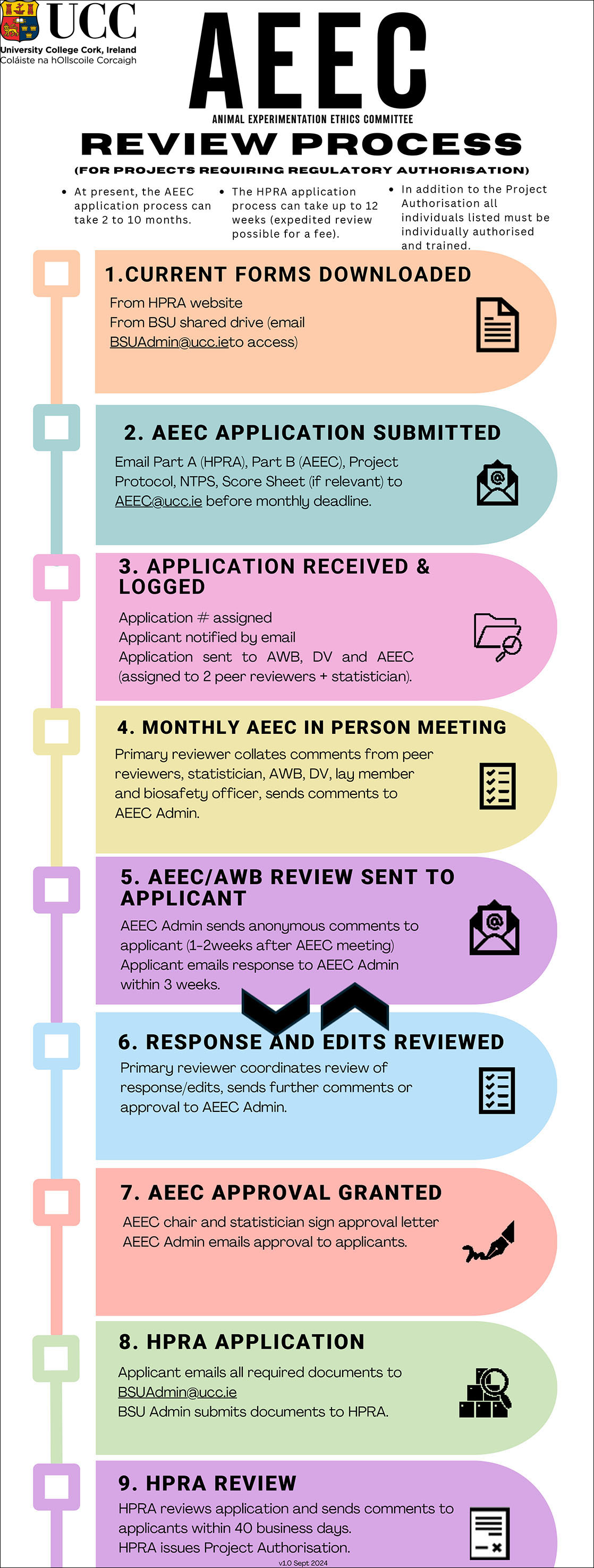
AEEC Review Process
There are two separate review processes:
- Application for the purpose of postmortem tissue collection.
- For projects requiring regulatory authorisation.
If the full images does not appear correctly, you can download the file from the following links:
Social Research
Non-clinical research which involves human participants must be approved by the Social Research Ethics Committee (SREC) or a formally approved SREC sub-committee. Ethical review by SREC is required where the methodology is not clinical or therapeutic in nature and proposes to involve:
- Direct interaction with human participants for the purpose of data collection using research methods such as questionnaires, interviews, observations, focus groups etc.
- Indirect interaction with human participant for example using observation, web surveys etc.
- Access to, or utilisation of, data concerning identifiable individuals.
Please note, if a research protocol falls into both the jurisdictions of CREC and SREC, then the application should usually be referred to CREC. - Email contact: srec@ucc.ie
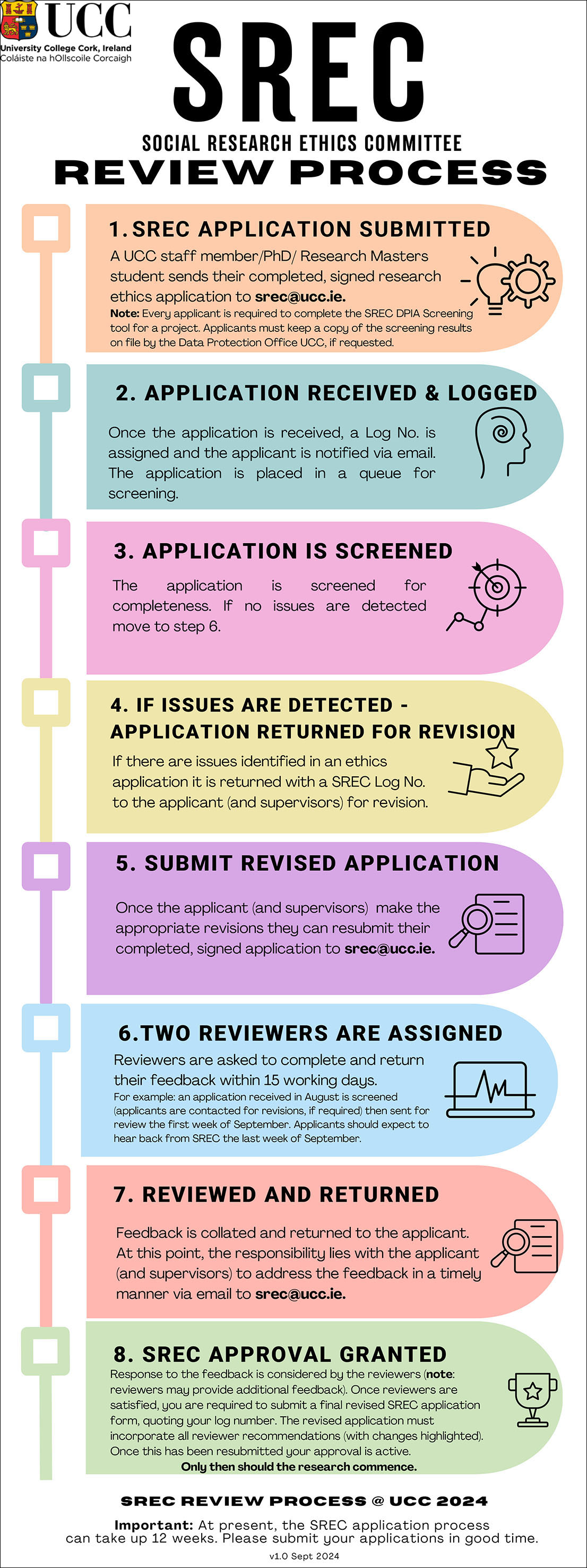
SREC Review Process
If the full image does not appear correctly, you can download the file from the following link:
UCC Research
Aistriú Taighde
Contact us
Office of Vice President for Research & Innovation, 4th Floor, Block E, Food Science Building University College Cork, T12 K8AF
