Ultraviolet Back in Time
by Bianca Govi, School of BEES, University College Cork
For me, as for everyone, the past 4 months have brought unexpected changes to work and research activities. Just as long-awaited lab supplies were finally delivered, the University was shut down as part of infection prevention measures. For months I had been planning lab work and developing protocols to generate the first set of data of my PhD. Then everything was put on hold. Semi-processed samples were abandoned on the bench, and plants left to wither in the growth room. I had to take a complete U-turn and convince my brain this was the time for reading at the desk.
My effort has by now developed into a meta-analysis attempt, using existing publications as my empirical data. The process of getting into this meta-analysis was challenging, and at times felt like herding cats… But focusing on publications as my raw material has given me the chance to ask questions about details of the narrative that I would not otherwise have devoted time to.
For instance, in the introduction sections of published UV papers, I have often come across references to how plant UV research started during the Ozone depletion crisis of the 1980s. To this, more recent papers add that a shift has now occurred in research focus, letting historical research slip into a limbo. This is because the effective withdrawal of CFC products has allowed for the stabilisation of the O3 layer. This major global victory of environmentally conscious legislation has (thankfully) made “worst case scenario” UV experiments now appear ecologically unrealistic.
After reading this sort of introductory remarks again and again, this began to feel like a less than ideal way of framing the legacy of past UV plant research. This presentation of past research felt both anticlimactic, and abrupt. Did the field really coalesce out of nothing because of the by-product of atmospheric pollution? If I was to contribute to the building, I needed to know where the foundation stone lay.
Another issue that pushed me to look further back in time was of the how and why of the common UV-A, UV-B, UV-C nomenclature. This was embraced by plant UV research in connection with ozone layer depletion, as the UV-B fraction is the one whose transmittance is altered due to O3 absorption. The distinctions between the three UV wavelength regions are, however, much older, and understanding the thinking behind the nomenclature felt necessary to appraise their usefulness in the description of “slippery” objects such as “invisible” wavelengths are.
So, I went digging as far back as I could, from within the walls of my room.
The first report of an effect of ultraviolet radiation on plants I could find dates back to 1894, and concerns crop damage from exposure to an unscreened carbon arc lamp. This apparatus would have emitted shortwave radiation, so the damage is not surprising. More interesting is the report of a microscopy observation of the damage inflicted to the cells of an aquatic plant after exposure to a beam of 2800 Å wavelength (or 280 nm, or shortwave UV-B), from 1904. Interest gathered and, in 1915, a worker from the Harvard Laboratory of Plant Physiology, published details on how to build a mercury vapour lamp intended for use in plant response experiments (Bovie, 1915).

Studies based on filtration of natural sunlight through different types of glass also emerged. Phototherapy glasses had been developed following the realisation that light quality is important for the production of vitamin D in animals, as well as for the treatment of other medical ailments. In parallel, plant UV research of the 1920s also investigated how to optimise the light spectrum for the improvement of plant health and vigour. So much so that a substantial body of work had accumulated by the end of the 1920s, but with contradicting results and debate on the function of ultraviolet. Different workers were equally strong in recommending UV-transparent or UV-opaque glass for greenhouses, see for example Delf (1928) and Popp and Brown (1933) for reviews. The application of UV glasshouses yielded mixed results and no solid consensus, so that very few researchers pursued these studies in the post-war years (Brodführer, 1955).
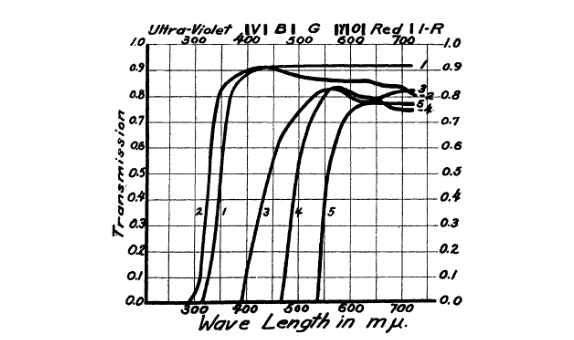
The popularisation of medical phototherapy is also what catalysed the establishment of the “ABC” system in ultraviolet nomenclature. In 1932, a meeting was held in Copenhagen (the Second International Congress on Light) at which the system was proposed for the standardisation of medical treatments (Coblentz, 1932). In those days, the spectral regions were not so strictly defined in terms of wavelength, as much as by which set of filters they could be isolated with. Interestingly, the company producing one of these nomenclature-defining filters (Noviol-0 glass), Corning Glass, is still in business, and produces a range of technical glasses and ceramics, including smartphone screens.

UV plant literature in the ’40s and ’50s is mostly characterised by studies on the use of shortwave “germicidal” UV lamps as a means for studying damage and repair mechanisms (termed “photo reversal” or “photoreactivation” as driven by visible light) (Bawden and Kleczkowski, 1952; Tanada and Hendricks, 1953). These wavelengths fall within the UV-C bracket, and are the highest energy ultraviolet wavelengths, strongly absorbed by many macromolecules including DNA (Caldwell, 1977). They are, however, not ecologically relevant as they do not reach the Earth’s surface thanks to interaction with the atmosphere. Some investigations also reported the degradation of auxin, but again using non-ecologically relevant UV-C wavelengths. An uncharacteristic stimulatory response of tobacco and sunflower plants under germicidal light is also reported (Koeppe et al., 1969). When germicidal light was dimmed due to passage through multiple window glass layer plants grew taller, had larger leaves and contained higher levels of chlorogenic acid and related compounds. Koeppe et al. (1969) mention their lamp also had an emission tail in 280-320 nm range. No spectral measurements were taken, but these longer wavelengths were likely less affected by the filtration, so that we might retroactively categorise this as a UV-B study.
It is in the 1970s that plant ultraviolet research began to focus its efforts on the sector of the spectrum denominated as UV-B. Such research became critical following the discovery of the thinning of the tropospheric ozone layer, and the realisation that this change in atmospheric chemistry influenced the amount of these mid-wavelength ultraviolet radiation reaching the planet’s surface. There was already some understanding of the ecological and evolutionary implication of ultraviolet adaptation in plant communities, mostly achieved by studying plants living in alpine environments (Caldwell, 1968) (Popp and Brown, 1933). Researchers now began to ask how ecosystems used to low UV-B irradiance would respond to a rapid intensity increase (Caldwell, 1971). After the successful enforcement of the Montreal Protocol (1989), the release of ozone-degrading anthropogenic compounds dropped, and ozone layer damage has now largely stabilised, although full recovery is still some time off (EEAP, 2019).
The focus on the effects of UV-B on plants has, however, endured, and been given new momentum by the discovery of a UV-B-specific photoreceptor (Rizzini et al., 2011) and UV-B specific gene transcription. The UVR8 (UV resistance locus 8) photoreceptor absorbance peak is within the traditional UV-B bracket and is so far the only known UV-B photoreceptor. However, serious questions remain about the activity of UVR8 in the UV-A part of the spectrum, as well as of UV-A sensors in the UV-B part of the spectrum, underlying that the somewhat artificial character of the distinction between these two wavelength areas.
To address this issue, an idea to be taken from early literature would be to go back to the pre-ABC system, where electromagnetic radiation was described in terms of wavelengths and not name tags. Challenging the use of the rigid spectrum subdivision and focusing on describing treatments on a quantitatively finer scale could aid the identification of biologically relevant patterns, making conceptual frameworks closer to the electromagnetic wavelength bands as perceived by plants.
References
Bawden, F.C., Kleczkowski, A., 1952. Ultra-Violet Injury to Higher Plants Counteracted by Visible Light. Nature 169, 90–91. https://doi.org/10.1038/169090a0
Bovie, W.T., 1915. Simple Quartz Mercury-Vapor Lamps for Biological and Photochemical Investigations. J. Biol. Chem. 20, 315–320.
Brodführer, U., 1955. Der Einfluss Einer Abgestuften Dosierung von Ultravioletter Sonnenstrahlung auf das Wachstum der Pflanzen. Planta 45, 1–56. https://doi.org/10.1007/BF01937677
Caldwell, M.M., 1977. The Effects of Solar UV-B Radiation (280–315 nm) on Higher Plants: Implications of Stratospheric Ozone Reduction, in: Castellani, A. (Ed.), Research in Photobiology. Springer US, Boston, MA, pp. 597–607. https://doi.org/10.1007/978-1-4613-4160-4_62
Caldwell, M.M., 1968. Solar Ultraviolet Radiation as an Ecological Factor for Alpine Plants. Ecol. Monogr. 38, 243–268. https://doi.org/10.2307/1942430
Coblentz, W.W., 1932. The Copenhagen Meeting of the Second International Congress on Light. Science 76, 412–415.
Delf, E.M., 1928. The Influence of Ultra-Violet Light on Plants. Biol. Rev. 3, 261–269. https://doi.org/10.1111/j.1469-185X.1928.tb00608.x
Koeppe, D.E., Rohrbaugh, L.M., Wender, S.H., 1969. The effect of varying u.v. intensities on the concentration of scopolin and caffeoylquinic acids in tobacco and sunflower. Phytochemistry 8, 889–896. https://doi.org/10.1016/S0031-9422(00)85879-3
Popp, H.W., 1926. A Physiological Study of the Effect of Light of Various Ranges of Wave Length on the Growth of Plants. Am. J. Bot. 13, 706–736. https://doi.org/10.2307/2435475
Popp, H.W., Brown, F., 1933. A Review of Recent Work on the Effect of Ultraviolet Radiation Upon Seed Plants. Bull. Torrey Bot. Club 60, 161–210. https://doi.org/10.2307/2480419
Rizzini, L., Favory, J.-J., Cloix, C., Faggionato, D., O’Hara, A., Kaiserli, E., Baumeister, R., Schäfer, E., Nagy, F., Jenkins, G.I., Ulm, R., 2011. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 332, 103–106. https://doi.org/10.1126/science.1200660
Tanada, T., Hendricks, S.B., 1953. Photoreversal of Ultraviolet Effects in Soybean Leaves. Am. J. Bot. 40, 634–637. https://doi.org/10.2307/2438452
