Past Projects
On This Page
Ambident Reactivity of Diazine N-Oxides
Reference: Chem. Sci. 2020, 11, 9630
The most popular means of rationalising the outcomes of reactions of ambident nucleophiles with electrophiles is “Hard-Soft Acid-Base” (HSAB) theory. The preferred site of alkylation of diazine N-oxides (ambident nucleophiles) by representative hard and soft alkylating agents was established conclusively using the 1H-15N HMBC NMR technique in combination with other NMR spectroscopic methods. Alkylation of pyrazine N-oxides occurs preferentially on nitrogen regardless of the alkylating agent employed, while O-methylation of pyrimidine N-oxide is favoured in its reaction with MeOTf. As these outcomes cannot be explained in the context of the hard/soft acid/base (HSAB) principle, we have instead turned to Marcus theory to rationalise these results.
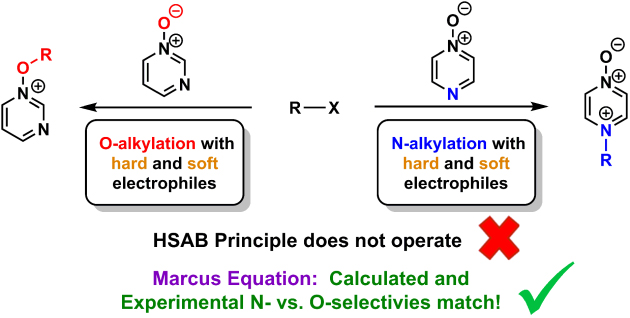
Methylation reactions of pyrazine N-oxide and pyrimidine N-oxide by MeI and MeOTf were investigated computationally (collaborator Dr Martin Breugst, Universität zu Köln), and used to derive Gibbs energies of activation (∆G‡) for the processes of N- and O-methylation, respectively. These values, as well as those derived directly from the DFT calculations, closely reproduce the observed experimental N vs O selectivities for methylation reactions of pyrazine N-oxide and pyrimidine N-oxide, indicating that Marcus theory can be used in a semi-quantitative manner to understand how the activation barriers for these reactions are constructed. It was found that N-alkylation of pyrazine N-oxide is favoured due to the dominant contribution of ∆rG° to the activation barrier in this case, while O-alkylation of pyrimidine N-oxide is favoured due to the dominant contribution of the intrinsic barrier (∆G0‡) for this process. These results are of profound significance in understanding the outcomes of reactions of ambident reactants in general.
Ambident Reactivity of Carbonyl-Stabilised Phosphonium Ylides
Reference: J. Am. Chem. Soc. 2016, 138, 11272
The most popular means of rationalising the outcomes of reactions of ambident nucleophiles with electrophiles is “Hard-Soft Acid-Base” (HSAB) theory. Carbonyl-stabilised phosphonium ylides are archetypal ambident nucleophiles. By measuring the rate constants (k) of the reactions of such ylides at the O and C-sites with benzhydrylium ions and using the Patz-Mayr equation (log10 k = s(E + N), where E is the electrophilicity of the electrophile, N is the nucleophilicity of the nucleophile, and s is a solvent-dependent nucleophile sensitivity parameter related to the extent of completion of bond formation in the transition state of the reaction), it was possible to establish that O-attack is always kinetically favoured, and that the product of C-attack is only observed when O-attack is reversible.
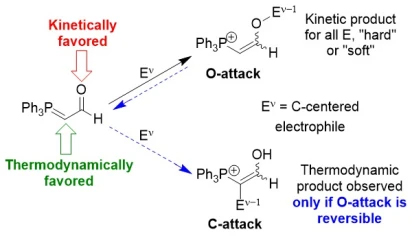
This conclusion contradicts the outcome predicted by HSAB theory. Combined with many other similar examples of the shortcomings of HSAB theory, the results show that the use of and in particular the teaching of HSAB theory as a means of rationalising ambident organic reactivity should be ceased.
Reactivity of Vinyl cations
Reference: J. Am. Chem. Soc. 2017, 139, 1499
The standard organic chemistry textbook rationale for the mechanism of the SN1 reaction posits that fast SN1 reactions are as rapid as they are due to the involvement of relatively stable carbocations. The implication of this rationale is that SN1 reactions that are slow or occur with negligible rate involve substantially less stable carbocation intermediates. A corollary of the above is that the “unstable” carbocation intermediates of these slow SN1 reactions should react exceptionally quickly with nucleophiles. This logic ignores the potential influence of the activation barrier on the reaction rate.
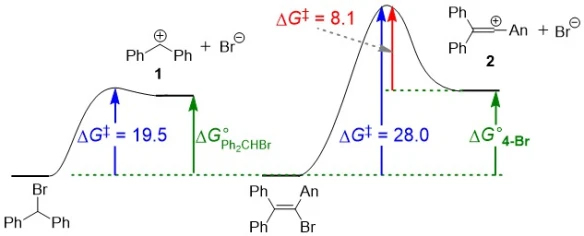
Vinyl cations are one of the classic examples of carbenium ions that have been assumed to be highly unstable due to the very low or non-existent rates of solvolysis reactions of the precursor vinyl halides. In order to probe whether the vinyl halide solvolyses are slow due to the high instability of the carbenium ion intermediate or due to a high activation barrier, the rates of the reactions of vinyl cations generated by laser flash photolysis with numerous nucleophiles were measured.
Benzhydrylium ion 1 (see Figure below) is ca. 106 more electrofugal than vinyl cation 2, i.e. solvolysis reactions that produce it are many orders of magnitude faster than those producing 2. Despite this, reactions of vinyl cations with nucleophiles are slower than the corresponding reactions of benzhydrylium ions of greater electrofugality, indicating that the slow formation of vinyl cations in vinyl halide solvolyses is due to a high activation barrier and not due to any particularly unusual instability of the carbenium ion product. Marcus theory is used to rationalise the results – the high activation barrier is primarily a consequence of a high intrinsic barrier ΔG0‡ for vinyl halide solvolysis.
High level quantum chemical calculations were used to corroborate that vinyl cations are not particularly unstable compared to other cations (calculated methyl anion affinities), and to show that high intrinsic barriers are responsible for the relatively slow heterolyses of vinyl halides and the slow nucleophilic additions to vinyl cations.
Identification of N‐ or O‐Alkylation of Aromatic Nitrogen Heterocycles and N‐Oxides Using 1H–15N HMBC NMR Spectroscopy
Reference: Eur. J. Org. Chem. 2020, 3270
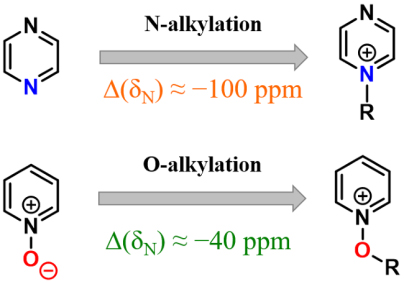
An analytical method based on 1H–15N, 1H–13C HMBC and 13C{1H} NMR spectroscopy has been developed that allows unambiguous diagnosis of the occurrence of N‐ or O‐alkylation of aromatic N‐heterocycles and N‐oxides. A systematic large upfield shift inthe 15N NMR chemical shift is shown to be characteristic of N‐alkylation of aromatic N‐heterocycles (Δ(δN) ≈ –100 ppm). A much smaller systematic change in δN is indicative of N‐oxide O‐alkylation (ca. –40 ppm).
Demonstration of the Operation of a Common Mechanism in All Li-salt Free Wittig Reactions
References: J. Am. Chem. Soc. 2012, 134, 9225, Tetrahedron Lett. 2012, 53, 6701, Chem. Soc. Rev. 2013, 42, 6670
Since the discovery of the Wittig reaction in 1953, its mechanism has never been definitively settled, with many different variants proposed and disproved. The first proposal that gained widespread acceptance involved initial formation of a betaine followed by ring closure to oxaphosphetane (OPA) and subsquent cycloreversion of OPA to give the products. This mechanism has since fallen into disfavour, to be replaced by the [2+2] cycloaddition mechanism, whereby OPA is formed directly and irreversibly from ylide + aldehyde. The stereochemistry of the alkene is thus decided in the OPA-forming step. Several of the developments that led to the consensus on this mechanism occurred many years apart from each other. This fact, along with the poorly understood effects of Li cation on the reaction, and the differing selectivities observed in the reactions of different types of ylide have led to confusion on the issue in the wider world of chemistry, which is reflected in the content of many undergraduate textbooks. The viewpoint persists that different mechanisms operate in different Wittig reactions, with some involving reversible formation of OPA or even involving betaine.
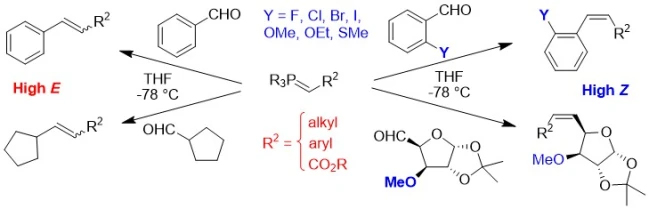
The basis of this project was the discovery of an effect that operates in representative examples of all types of phosphonium ylide under Li salt-free conditions. Reactions of both aromatic and aliphatic aldehydes bearing a heteroatom substituent in the β-position relative to the carbonyl group uniformly show dramatically increased selectivity for Z-alkene or its precursor, cis-OPA, compared with analogous aldehdyes lacking such a substituent (see Scheme above). The origin of the effect lies in the existence of a bonding interaction between the phosphorus and the β-heteroatom in the cycloaddition transition state leading to the OPA intermediate. This is the first time that an effect that is common to all Wittig reactions has been discovered. Since it is consistent across all types of ylide and aldehyde, there must be a single mechanism in operation in these and therefore all other Li salt-free Wittig reactions. Furthermore, the effect can only be rationalised in the context of the direct cycloaddition mechanism, and so this work shows unequivocally, and for the first time, that all Wittig reactions occur by the same [2+2] cycloaddition mechanism under Li salt-free conditions. The issue of the mechanism of one of the most important reactions in organic chemistry has thus been thus settled.
New Method for Purification of the Alkene Product of the Wittig Reaction
Reference: Org. Biomol. Chem. 2012, 10, 3531
Purification of the alkene produced in a Wittig reaction is often a troublesome procedure, since the removal of the phosphine oxide by-product is far from straightforward. Even chromatographic removal of this by-product may not suffice if the alkene product is sensitive to isomerisation or decomposition on exposure to the chromatographic stationary phase or light. During the Wittig mechanism project described above, I developed a new method for the chromatography-free purification of the alkene products and regeneration of phosphine starting material. Thus, addition of oxalyl chloride to the crude product causes formation of chlorophosphonium salt (CPS, see Scheme below).
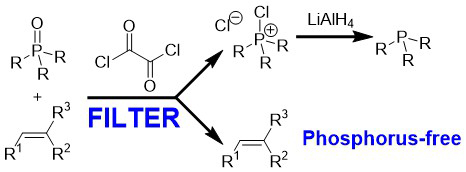
The alkene product can be dissolved in cyclohexane or cold diethyl ether and isolated from the insoluble CPS by simple filtration. The isolated CPS can be converted into phosphine in high yield by treatment with LiAlH4. This new method substantially contributes to the operational simplicity of the Wittig reaction, and can also be applied to the purification of the alkyl chloride products of the Appel reaction.
Mechanisms of Phosphonium Salt and Ylide Hydrolysis and Alcoholysis
References: Chem Commun. 2015, 51, 1147, Chem. Eur. J. 2016, 22, 9033
Many of the details of the mechanism of alkaline hydrolysis of phosphonium salts have long been established and understood, but the pivotal pentavalent phosphorane intermediate of the reaction had never been observed or characterised, despite strong indirect evidence pointing to its existence. Phosphonium ylides have been supposed to follow a virtually identical mechanism with one extra initial step – protonation of the ylide starting material to give phosphonium salt. Phosphonium salt and ylide alcoholsis were also supposed to follow analogous mechanisms.
As part of these projects, the only example of a tetraorganophosphorane intermediate was synthesised by low temperature reaction of a constrained cyclic ylide with H2O, and characterised by low temperature NMR. This compound breaks down to give the products expected of ylide/salt hydrolysis. Numerous tetraorganoalkoxyphosphoranes (from ylide + alcohol) were also synthesised, including one derived from a constrained ylide. By NMR spectroscopic observation of the behaviour of these phosphoranes, it was established that initial proton of the ylide is in many cases (especially in aprotic organic solvents) an impossible process, and that formation of these intermediates occurs by concerted addition of an O-H bond to the ylide P=C bond. In some instances of phosphonium salt hydrolysis and alcoholysis, the reaction may actually go through ylide on the way to phosphorane. The potential of the stable phosphorane intermediates to act as carbanion equivalents will be explored in the next step of this project.
Nucleophilicity and Lewis Basicity of nNicotine and Analogues
Reference: J. Phys. Org. Chem. 2016, 29, 759
Nicotine has two amine functional groups, and so in principle has the capacity to be an ambident nucleophile. In order to sort through some of the apparently contradictory results on its reacitivity in the literature, the rate and equilibrium constants (k and K, respectively) of reactions of nicotine and analogous amines with benzhydrylium ions were measured. The Patz-Mayr equation (see above) and the related thermodynamic equation (log10 K = LA + LB, where LA is the Lewis acidity of the benzhydrylium ion and LB is the Lewis basicity of the base (amine)) were used to establish the nucleophilicity and Lewis basicity of each amine. Astoundingly, the pyridyl N of nicotine was found to be the more nucleophilic and Lewis basic than the pyrrolidinyl N, despite the fact that the closely related compounds N-methylpyrrolidine and N-methyl-2-phenylpyrrolidine are significantly more nucleophilic and Lewis basic than either 3-methylpyridine or nicotine. Quantum chemical calculations were carried out to attempt to establish the cause of the deactivation of the pyrrolidine N in nicotine.
Investigation of the Occurrence of Stereochemical Drift in Wittig Reactions of Non-Stabilised Ylides
Reference: Eur. J. Org. Chem. 2014, 86
Isolated examples of Wittig reactions of non-stabilised ylides exhibit a marked difference between the diastereomeric ratio of the OPA intermediate and alkene product. In an attempt to observe this phenomenon in action, OPA decomposition was monitored by NMR spectroscopy, both at different temperatures, and over time at a constant temperature. It was demonstrated that this “stereochemical drift” is a general phenomenon in reactions of ethylides with benzaldehydes.